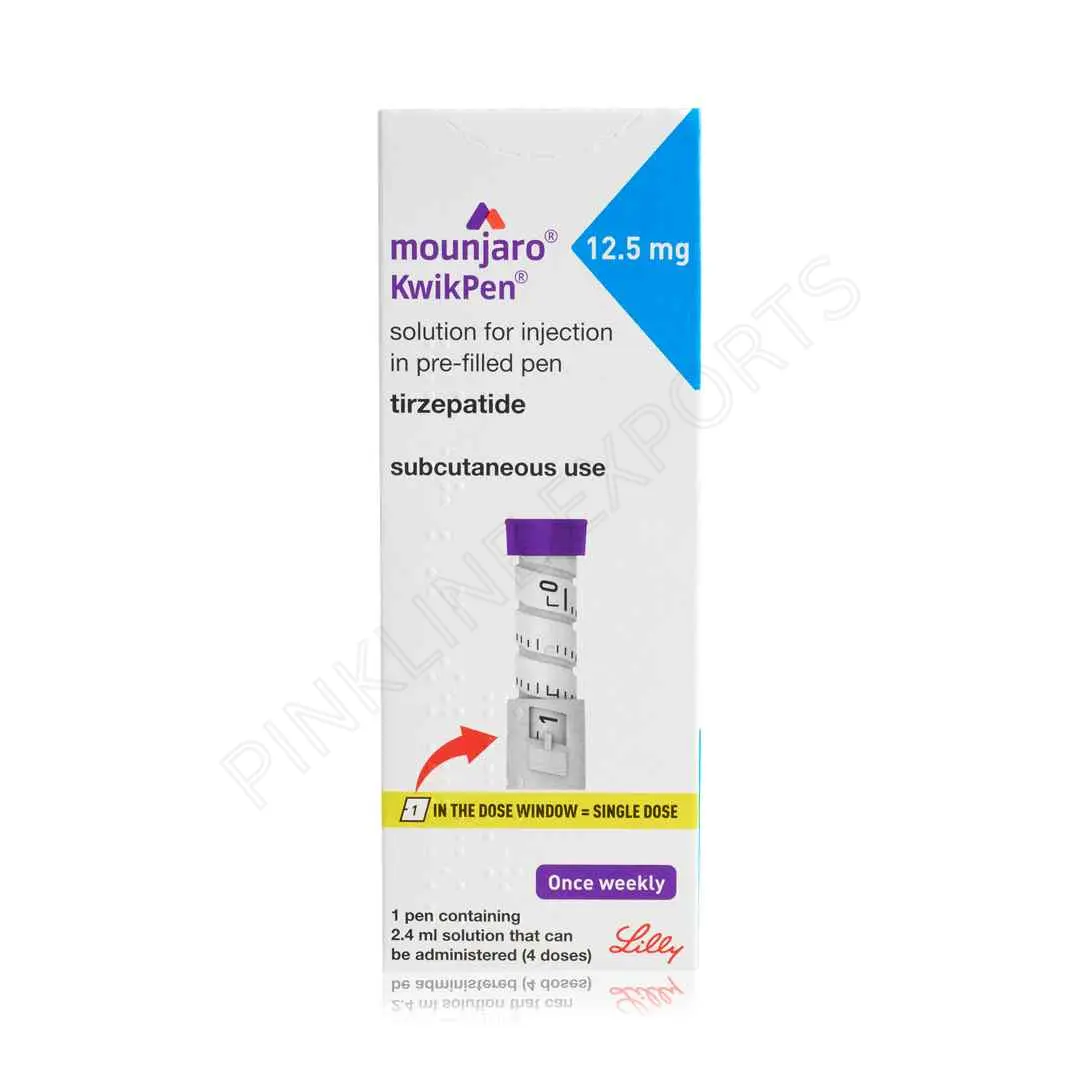
Mounjaro 12.5 Mg – Kwikpen
$380.00
Mounjaro 12.5 Mg – Kwikpen is a once-weekly injectable medication containing tirzepatide, prescribed for adults with type 2 diabetes who require advanced glycemic control. This dose is typically used after successful titration from lower strengths and prior to reaching the maximum recommended dose.
Description
Mounjaro 12.5 Mg – Kwikpen is a prescription injectable therapy developed to improve blood glucose control in adults with type 2 diabetes mellitus. It belongs to a novel class of medications known as dual incretin receptor agonists, targeting two key metabolic receptors:
-
Glucose-dependent insulinotropic polypeptide (GIP)
-
Glucagon-like peptide-1 (GLP-1)
By activating both pathways, tirzepatide offers a broader and more effective metabolic response compared to single-pathway incretin therapies.
The Kwikpen is a prefilled, fixed-dose injection device designed to simplify administration, ensure dosing accuracy, and support long-term treatment adherence.
Mechanism of Action
Tirzepatide mimics the activity of natural incretin hormones released after food intake. These hormones regulate glucose metabolism through several complementary mechanisms.
Mounjaro 12.5 Mg – Kwikpen works by:
-
Increasing insulin secretion in a glucose-dependent manner
-
Reducing glucagon release to limit excess glucose production by the liver
-
Delaying gastric emptying to reduce post-meal glucose spikes
-
Enhancing satiety, which may contribute to reduced caloric intake
This dual-receptor activity provides sustained and clinically meaningful glycemic control across the weekly dosing interval.
Who Should Use This Dose?
Mounjaro 12.5 Mg – Kwikpen is intended for:
-
Adults diagnosed with type 2 diabetes mellitus
-
Patients who have tolerated and progressed through lower-dose tirzepatide therapy
-
Individuals who require stronger glycemic control but are not yet indicated for the highest dose
This dosage is not suitable for treatment initiation and should only be prescribed after medical evaluation and supervised dose escalation.
Dosage and Administration
-
Recommended Dose: 12.5 mg once weekly
-
Route: Subcutaneous injection
-
Injection Sites: Abdomen, thigh, or upper arm
-
Dosing Schedule: Same day each week
Injection sites should be rotated to minimize local reactions. Patients should follow healthcare provider instructions carefully and should not alter the dose without medical guidance.
Clinical Benefits
Patients prescribed Mounjaro 12.5 Mg – Kwikpen may experience:
-
Significant reduction in fasting and postprandial blood glucose levels
-
Improved HbA1c control compared to lower doses
-
Once-weekly dosing convenience
-
Dual incretin activity for comprehensive metabolic regulation
-
Potential weight reduction when combined with dietary and lifestyle modifications
Clinical outcomes vary and require regular monitoring by a healthcare professional.
Safety Information and Warnings
Before starting Mounjaro 12.5 Mg – Kwikpen, patients should inform their healthcare provider of any current or past medical conditions.
Use with caution in patients with:
-
History of pancreatitis
-
Medullary thyroid carcinoma
-
Multiple endocrine neoplasia syndrome type 2
-
Severe gastrointestinal disorders
Common Side Effects
-
Nausea
-
Vomiting
-
Diarrhea
-
Constipation
-
Decreased appetite
These effects are commonly reported during dose escalation and may diminish over time.
Serious Adverse Reactions
Immediate medical attention is required if severe abdominal pain, persistent vomiting, or signs of hypersensitivity occur.
Drug Interactions
Mounjaro may delay gastric emptying, which can affect the absorption of orally administered medications.
Caution is advised when used alongside:
-
Insulin
-
Sulfonylureas
-
Medications with a narrow therapeutic index
Dose adjustments and clinical monitoring may be necessary.
Storage and Handling
-
Store refrigerated at 2°C to 8°C before first use
-
Do not freeze
-
Protect from excessive heat and direct light
-
Follow healthcare provider instructions for in-use storage
-
Keep out of reach of children
Frequently Asked Questions (FAQs)
1. What is Mounjaro 12.5 Mg – Kwikpen used for?
It is used to improve blood sugar control in adults with type 2 diabetes who require advanced tirzepatide therapy.
2. Is this the maximum dose?
No. The maximum approved dose is 15 mg.
3. Can new patients start at this dose?
No. Treatment must begin at lower doses and be gradually increased.
4. How often is it injected?
Once weekly.
5. Does it increase the risk of hypoglycemia?
Risk is low when used alone but increases when combined with insulin or sulfonylureas.
6. Is it approved for weight loss?
It is approved for diabetes management, though weight reduction may occur as a secondary effect.
7. Can I change my injection day?
Yes, provided at least 72 hours have passed since the last dose.
8. What if I miss a dose?
If within 4 days, take it as soon as possible. Otherwise, skip and resume the next scheduled dose.
9. Does it require refrigeration?
Yes, refrigeration is required before first use.
10. Who should avoid using this medication?
Patients with type 1 diabetes or specific thyroid-related conditions should not use it.
Conclusion
Mounjaro 12.5 Mg – Kwikpen is an advanced-dose tirzepatide therapy designed for adults with type 2 diabetes who require enhanced glycemic control after progressing through lower doses. Backed by clinical evidence and prescribed under medical supervision, it offers sustained metabolic benefits through its dual GIP and GLP-1 receptor activity, supporting long-term diabetes management.

.svg)









Reviews
There are no reviews yet.