Ozempic vs Rybelsus (2026): Differences, Effectiveness, Safety & Weight Loss

Ozempic vs Rybelsus: Injectable vs Oral Semaglutide — Evidence-Based Comparison (2026)
Dr. Antonino Belfior, MD
Specialty: Endocrinology / Internal Medicine
Resources : PubMed,Frontiers Article,National Center for Biotechnology Information (NCBI)
This article has been medically reviewed for clinical accuracy, evidence-based integrity, and patient safety. The information reflects current peer-reviewed research and medical guidelines available as of January 2026.
Educational Disclaimer:
This content is intended for informational purposes only and does not substitute professional medical advice, diagnosis, or treatment. Always consult a qualified healthcare provider before starting, stopping, or changing any medication, including Ozempic or Rybelsus.
Semaglutide has emerged as one of the most impactful therapies for type 2 diabetes mellitus (T2DM) and medically supervised weight management. Two widely prescribed formulations—Ozempic (injectable semaglutide) and Rybelsus (oral semaglutide)—contain the same active molecule but differ significantly in administration, absorption, and clinical outcomes.
What Is Semaglutide?
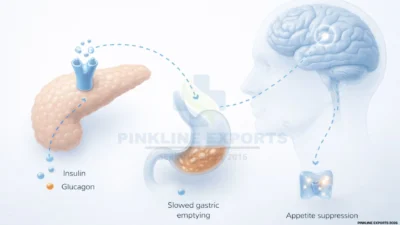
Semaglutide is a GLP-1 (glucagon-like peptide-1) receptor agonist that improves metabolic control by:¹
Enhancing glucose-dependent insulin secretion
Suppressing glucagon release
Slowing gastric emptying
Reducing appetite and caloric intake
Extensive clinical programs (SUSTAIN and PIONEER trials) confirm its dual benefit in glycemic control and weight reduction.
Ozempic vs Rybelsus: Key Differences
| Feature | Ozempic | Rybelsus |
|---|---|---|
| Administration | Subcutaneous injection | Oral tablet |
| Dosing | Once weekly | Once daily |
| Bioavailability | High, consistent | Low, variable |
| Weight-loss effect | Higher | Moderate |
| Absorption dependency | Injection site | Empty stomach |
Pharmacokinetic studies show injectable semaglutide provides more predictable systemic exposure, explaining its stronger clinical outcomes.
Ozempic (Injectable Semaglutide)
Ozempic is administered once weekly and has been extensively studied in the SUSTAIN trial program.
Clinical Benefits
Significant HbA1c reduction¹
Greater average weight loss than oral semaglutide
Proven cardiovascular risk reduction in high-risk patients
Improved adherence due to weekly dosing
Clinical Considerations
Requires injections
Gastrointestinal side effects during dose escalation
Cold-chain handling may be needed in some regions

Rybelsus (Oral Semaglutide)
Rybelsus is the first oral GLP-1 receptor agonist, approved following the PIONEER clinical trial program.
Clinical Benefits
Needle-free administration⁵
Effective HbA1c reduction versus placebo and some oral antidiabetics
Suitable for early or moderate T2DM management
Limitations
Must be taken on an empty stomach with limited water
Lower and variable absorption compared to injections
Daily strict dosing requirements may affect adherence
Effectiveness: What Does the Evidence Show?
A 2024 systematic meta-analysis comparing oral and subcutaneous semaglutide found:
Greater HbA1c reduction with injectable semaglutide
Higher average weight loss with Ozempic
Comparable overall safety profiles
Clinical pharmacokinetic data further support these findings, demonstrating more consistent therapeutic exposure with injectable semaglutide.
Safety & Side Effects
Common (Both Forms)
Nausea
Vomiting
Diarrhea
Constipation
Rare but Serious
Pancreatitis
Gallbladder disease
Potential thyroid C-cell tumor risk (contraindicated in specific patients)
Both medications show similar safety profiles when used under medical supervision.
Who Should Choose Which?
Ozempic May Be Preferred If:
Weight loss is a primary treatment goal
Advanced glycemic control is required
Weekly dosing improves adherence
Rybelsus May Be Preferred If:
Needle avoidance is essential
Early-stage diabetes management is sufficient
Daily oral routines suit patient preference
Commonly Asked FAQ’s
Ozempic and Rybelsus both contain semaglutide, but Ozempic is a once-weekly injectable medication, while Rybelsus is taken orally once daily. The main difference lies in the route of administration, absorption, and clinical effectiveness.
Clinical studies show that injectable semaglutide (Ozempic) generally leads to greater reductions in HbA1c and more significant weight loss compared to oral semaglutide (Rybelsus), primarily due to more consistent absorption.
Rybelsus may lead to weight loss as a secondary benefit, but it is not as effective as Ozempic for weight reduction. Weight loss outcomes with Rybelsus are typically more modest.
Ozempic is generally considered more effective for weight loss than Rybelsus, based on clinical trial data showing higher average weight reduction with injectable semaglutide.
Both medications have similar safety profiles when used as prescribed. Common side effects include nausea, vomiting, and diarrhea. Neither is considered inherently safer; suitability depends on individual patient factors.
Switching between Rybelsus and Ozempic may be possible under medical supervision. Dose adjustments and timing considerations are important, so patients should consult a healthcare provider before switching.
Common side effects include nausea, vomiting, diarrhea, constipation, and reduced appetite. These effects are usually temporary and occur most often during dose escalation.
Conclusion
When comparing Ozempic vs Rybelsus, clinical evidence consistently supports injectable semaglutide as the more potent option, particularly for weight loss and long-term metabolic outcomes. However, oral semaglutide remains a valuable alternative for patients prioritizing non-injectable therapy.
Both formulations are FDA-approved, extensively studied, and clinically validated for use in 2026 when prescribed appropriately.

.svg)



